As mentioned earlier, early vitrification experiments have been performed in the traditional vessels of cryopreservation, i.e. 0.25 ml plastic insemination straws or cryovials. These tools were not designed for the special purpose, had a thick wall and required a relatively large amount of solution for safe loading (5-7 µl for the straw, and 10+ µl for cryovials). Accordingly, the theoretically achievable cooling and warming rates were quite limited (approximately 2000⁰C/min for straws, and much less for cryovials – fortunately, the latter vessels were forgotten soon). To avoid collapse or explosion of straws, we used two-step cooling and also warming – these manipulations, however, added more inconsistencies to the procedure. Moreover, the inequal solidification of these large volumes under extreme pressure changes resulted in cracking of the vitrified medium and fractures in the samples inside. So, instead of eliminating the inefficiency of traditional freezing, we preserved most handicaps and added more. Obviously, new devices were needed.
Thanks to the fabulous creativity of mammalian embryologist, nothing has happened for more than ten years, almost all “experts” accepted these limits and handicaps. Well, I have to admit – I also played a significant role in this impotence, for seven long years.
Of course, there were some preliminary attempts, for example, dropping embryos directly (without any holding tool) into the liquid nitrogen (Lauda and Tepla, 1990). Great idea, a pity that it does not work – the very first attempt should prove it. To form a drop from the concentrated vitrification solution minimum 5 µl solution is needed – far too much to achieve an appropriate cooling rate. Moreover, once the drop reached the surface of the liquid nitrogen, it started to float and made bizarre cruises on the surface for several (rather long) seconds. The reason is quite simple: the liquid nitrogen is just at its boiling point, at -196⁰. Anything warmer (like our drop with the sample) induces extensive evaporation and the created vapour coat functions as a great thermo-insulating layer, and also prevents the drop from sinking. The result is a miserable cooling rate that cannot even be correctly measured.
Well, papers based on this triple-handicapped system were repeatedly published for a dozen of years.
Another approach was the minimum drop size method (Arav, 1992) – an obvious but still remarkable idea. Unfortunately, it required expensive cryo-microscopes, and could not be used in everyday practice.
Eventually , the first method that has fully utilised the enormous potential of the small sample-direct contact approach was the application of copper electron microscopic grids as carriers (Martino et al., 1996). In fact, it was one of the best solutions, so far – the amount of the solution was low (although the standardisation and consistency was a problem), the thermo-conductive metal increased the cooling and warming rates, and the rapidly solidified solution film kept the sample safely on the surface. Removal after warming was also easy and safe. The small metal grid was a game-changer – opened the road to decrease the concentration of cryoprotectants and to exploit the practical value of the small-volume, direct contact approach.
However, the tiny carrier, the lack of method for proper identification and practical storage restricted the application to experimental laboratories.
We have to understand: embryologist preferred to work with straws. Everything, or almost everything in an embryo lab was cryopreserved in straws, early embryos, blastocysts, semen, oocytes (of which only a handful survived the old procedures. All Dewars, all canisters were designed for straw storage. They did not want to discard the whole infrastructure for a challenge to enter a new territory.
So, if they wanted straws, we provided them straws – with some modification – the OPS, the first purpose-designed tool for small volume – high cooling-warming rate vitrification (Vajta et al., 1998).

The benefits were obvious. Easy handling, just like a pen. Easy loading with a pre-defined standard volume, by using a drop and the capillary effect. No attachment to the surfaces, no distortion, as the sample floats in the solution column all the time. No danger of osmotic shock after loading, the solution column and the tube prevents it. Cooling and warming rates that allowed - despite the relatively robust and safe structure – low cryoprotectant concentrations and excellent survivals of embryos and oocytes of many mammalian species including humans. Easy storage in conventional Dewars and canisters.Easy expelling by using the warming – expanding air in the straw to push out slowly the solution. Easy and stress –free recovery of the vitrified sample from the warming dish.
According to independent opinions, learning and consistently using the OPS for a beginner embryologist is easier than that of any other high-speed vitrification methods designed and commercialised in the past 22 years. And, more importantly, none of the techniques introduced after the OPS offered better survivals, more consistent results or broader areas of applicability. The original is still the best.
Maybe, it is just pure luck – but it is so.
Of course, there are alternatives. More than supposed. Some estimations say there are at least 100 methods for high-speed vitrification today. There were years when almost everybody dealing – even marginally – with vitrification established a special vitrification device and technique, not speaking about personal variations of followers. Creation was only part of the problem, the second – may be even more challenging – task was the name. “Cryo”- and “Vitri”- were definitely abused, with extreme variations of endings: leafs, tops, locks, tips, safes, ingas, etc., etc. Funny modifications of the OPS were also introduced, with glass micropipettes (pls. note it is illegal to use glass for cryopreservation in many countries!), gel loading tips, closed-pulled straws, sterile stripper tips, pipetting tips – just a few examples.
Which one is the winner?
If we see the quantity, the winners are the ones that had the strongest financial support. They had the money for patenting, accreditations, advertising, marketing.
If we regard scientific acknowledgement, the picture is different. The original description of the OPS (Vajta et al., 1998) is still the most cited publication among papers dealing with vitrification. It was widely used in domestic animal embryology and for experimental purposes. Almost all cloned animals of the world born after embryo cryopreservation were vitrified with the OPS method (please note: cloned embryos are extremely fragile and sensitive to any interventions). As mentioned earlier, OPS produced the first human baby after oocyte vitrification (an approach that subsequently changed the whole world of human ART), and it is still the most efficient method for human embryonic stem cell vitrification.
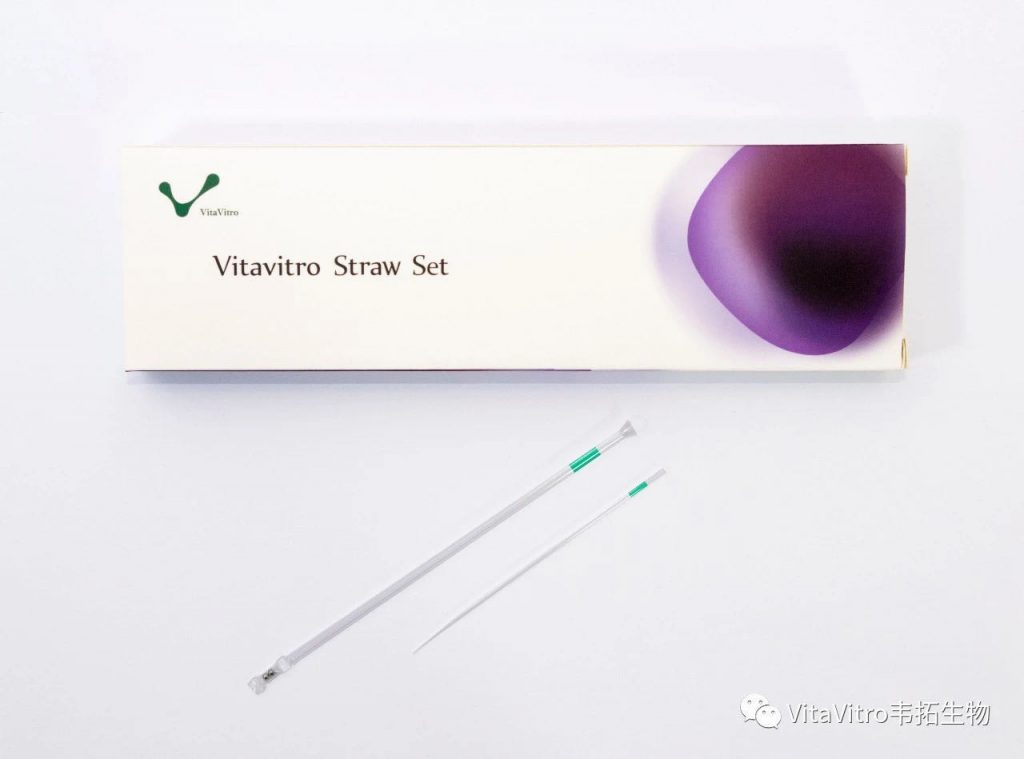
Well, the commercialisation of the OPS was delayed. I am not a businessman, and, until recently, was unlucky with my attempts to find a company that supports my innovations.
Finally, however, I became extremely lucky. VitaVitro’s team provides all that this technology deserves. Trust, administrative and financial support, creativity and production by using the highest standards in the world, great marketing and friendly, optimistic atmosphere.
OPS is now produced by VitaVitro and has obtained FDA and CE certifications. Also it has successfully certified by the extremely strict Chinese FDA for domestic and global sales.
And, of course, this is just the beginning.
For further reading:
Vitrification in ART: past, present, and future, Vajta G, Theriogenology 2020 (in press)
Vitrification in human and domestic animal embryology: work in progress, Vajta G, Reproduction, Fertility and Development 2013 25:719-27

